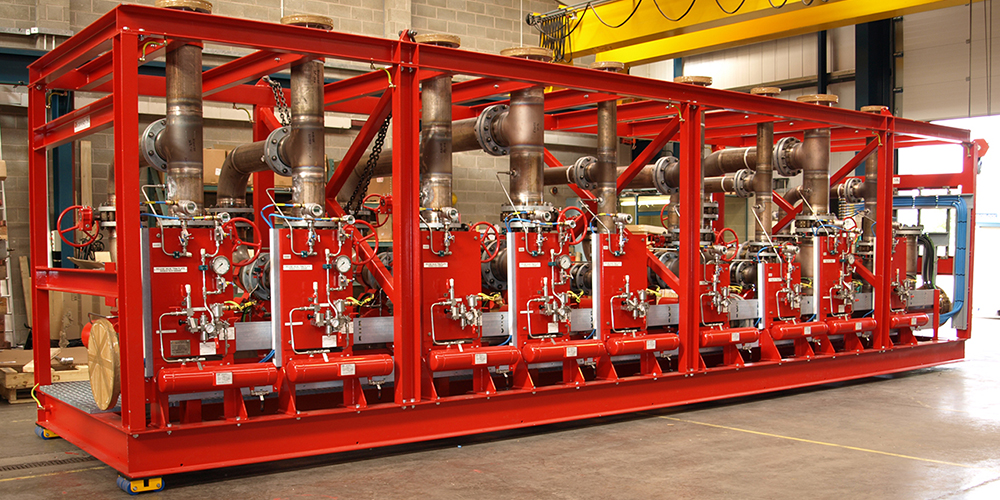
Safety is our First Priority
About Petroyaz FZE
Petroyas Group of Companies whilst having the benefit of having around 2 decades of experience is now a well- established leading international company in the field of Mechanical, Electrical & Electronic Engineering. The company’s entire history since its inception in 1996 has been an ongoing process of growth and expansion.
Petroyas Group of Companies is one of the major vendors to Oil & Gas Industries, Commercial & Residential Buildings, Industrial and Logistical Warehouses, Oil production Plants, Petro Chemical Plants & Power Plants.
This unique position having been achieved in such a short span, not only due to the aggressive marketing approach adopted by the staff and management of the company but also due to the excellent after sales services which have frequently been acknowledged and appreciated by many of its customers.
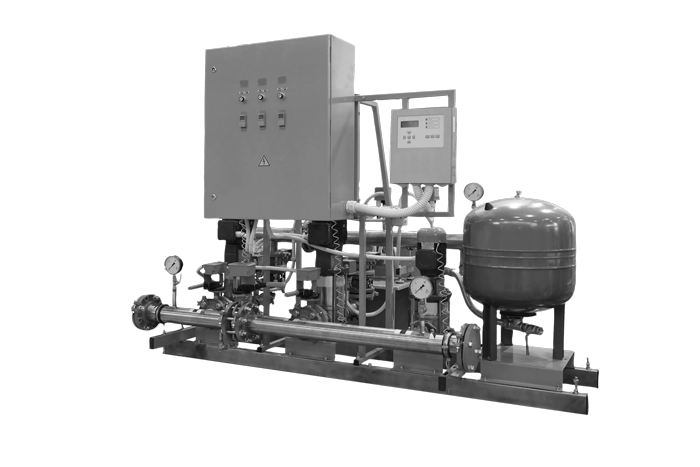
Services - Fire Detection System
We are having a wide range of international products with a dedicated sales team coverage in Middle East Region & European Region.Production & manufacturing
Production facilities are located at Dubai Free Zone occupied with all latest equipment’s, the production process is under the thorough monitoring & supervision of high experienced engineering technical team.Our Projects
Services - Fire Fighting System
We are having a wide range of international products with a dedicated sales team coverage in Middle East Region & European Region.Looking for our Services
Contact Now
Contact us or send a request for our services.